Regulation of neuronal excitability in physiological and pathological conditions


Regulation of neuronal excitability in physiological and pathological conditions
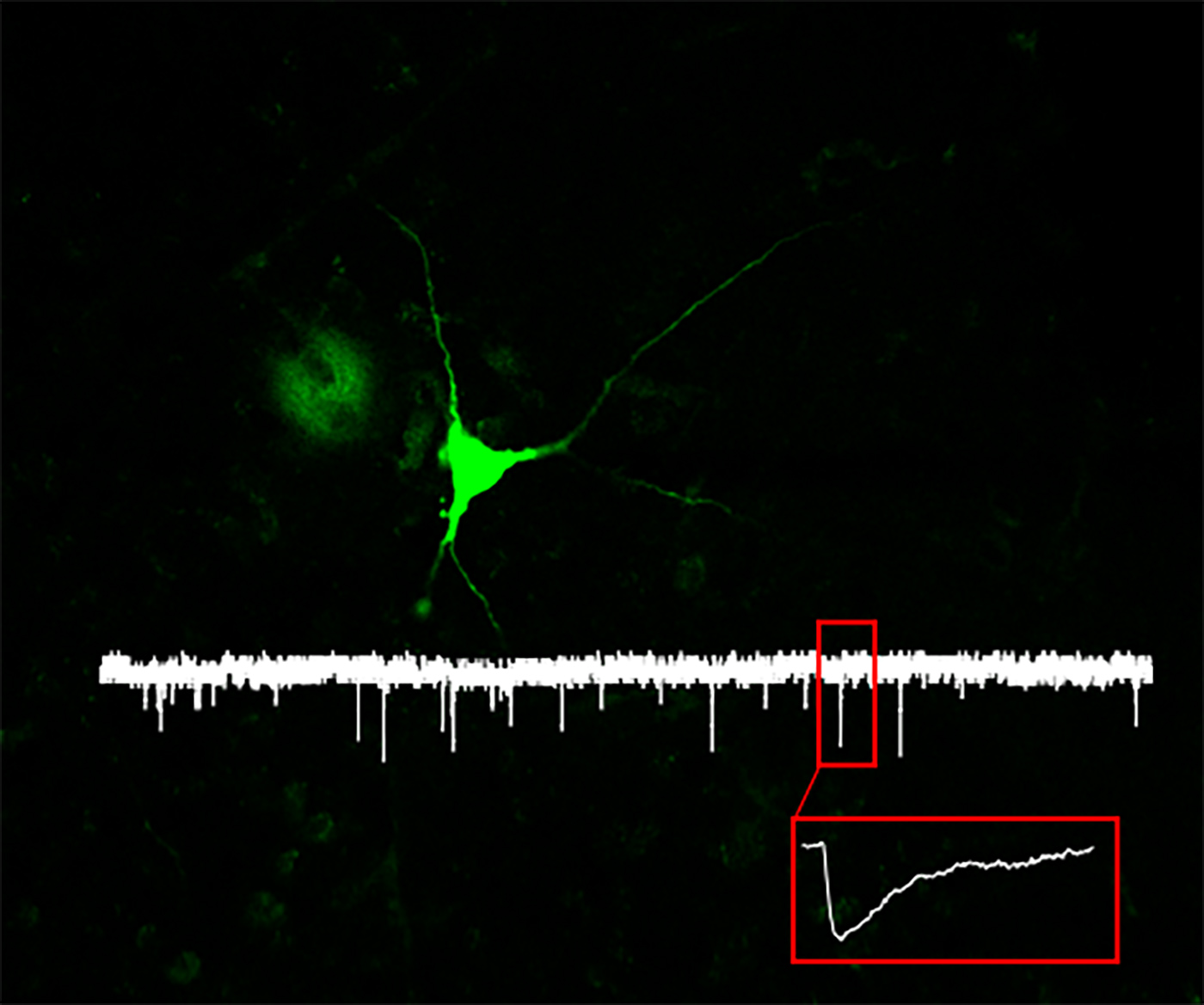
Brains process information and encode new stimuli through the activity of the underlying neuronal circuits. The balance between excitatory and inhibitory signals within these circuits is essential and its dysregulation has been linked to cognitive decline, psychiatric disorders and epilepsy. We seek to understand the mechanisms underlying these processes both in physiological and pathological conditions. To this end, we combine ex vivo electrophysiology with viral mediated gene transfer, molecular biology and behavior to study excitatory and inhibitory neuronal transmission in several brain areas, with a particular focus on the hippocampus.
The main lines of research of our laboratory are the following:
- Neuronal Excitability in Alzheimer’s Disease (AD)
While the most salient characteristics of AD is memory decline, electroencephalographic (EEG) recordings in brains of AD patients, people at high risk for AD, and of murine transgenic models have revealed altered electrical signaling well before the onset of cognitive decline, suggesting that modifications in neuronal electrical activity is detectable even at pre-symptomatic stages. We seek to understand what are the neuronal excitability properties that are altered in these early phases, how they relate to cognitive decline, and the underlying molecular mechanisms.
- Role of neuroligins in neurodevelopmental disorders
Neuroligins are a family of postsynaptic cell adhesion molecules that regulate stabilization and function of both excitatory and inhibitory synapses. A number of neurodevelopmental disorders are associated to mutations in genes coding for these proteins. To better understand this link, we are currently investigating how mutations in neuroligins lead to altered hippocampal synaptic plasticity and circuit function during development. At this stage, neuronal activity drives hippocampal circuit refinement, thereby influencing circuit wiring and long-term hippocampal function. With our work, we hope to clarify the cellular basis of neurodevelopmental learning delays.
Selected Publications
Rizzello E., Middei S., Marchetti C. “Synaptic Correlates of Anterograde Amnesia and Intact Retrograde Memory in a Mouse Model of Alzheimer’s Disease”. Current Alzheimer Research., 2020. PubMed
D’Amelio M, Cavallucci V, Middei S., Marchetti C., Pacioni S., Ferri A., Diamantini A., De Zio D., Carrara P., Battistini L., Moreno S., Marie H., Bacci A., Ammassari-Teule M., and Cecconi F. Non-apoptotic caspase-3 activity triggers synaptic degeneration at the onset of Alzheimer’s Disease. Nature Neuroscience.14: 69-76, 2011. PubMed
Marchetti C., Tafi E., Middei S., Rubinacci MA., Restivo L., Ammassari-Teule M., Marie H. Synaptic Adaptations of CA1 Pyramidal Neurons Induced by a Highly Effective Combinational Antidepressant Therapy. Biological Psychiatry. 67: 146-54, 2010. PubMed
